Etos-Scientific Corporate Responsibilities
Diversity
Etos-Scientific values diversity in our people and the organizations we deal with. At Etos-Scientific, business activities are conducted without discrimination based on race, color, genetics, religion, gender, gender identity or expression, sexual orientation, national origin, disability, age or status as a special disabled veteran or other veteran covered by the Vietnam Era Veterans Readjustment Act of 1974, as amended.
The design and administration of Etos-Scientific benefit plans comply with all applicable local laws where we perform these business activities, including those dealing with equal opportunity and affirmative action. In respecting and valuing the diversity among our employees and all those with whom we do business, managers are expected to ensure that there is a work environment free of all forms of discrimination and harassment.
Effective management of our workforce diversity policy is an important strategic objective. Every Etos-Scientific employee and supplier is expected to abide by this policy and uphold the company's commitment to workforce diversity.
These include compliance with the following regulations in the United Kingdom:
- Sex Discrimination Act 1995
- Disability Discrimination Act 1995
- Equal Pay Act 1975
- Race Relations Act 1976
- Commission for Racial Equality Code of Practice for Employment
Environment Policy
Etos-Scientific is committed to protecting the environment and controlling the usage of resources in manufacturing and related activities through continuous improvement of environmental protection as well as complies with relevant environmental legislation rules and regulations. Our environmental management systems define objectives and targets for prevention and reduction of pollution in manufacturing, and related processes to safeguard adverse impact on the environment. Even though we are a global company, we strive to minimize our carbon footprint through more efficient use of resources local to our customers and technology in managing of our worldwide operations.
Etos-Scientific is committed to protecting the environment. The environmental impact of any product and its packaging is decided upon in the design stage. We strive to develop designs which take into account the environmental impact of a product or packaging across its entire existence, from raw materials, manufacture, distribution, use and end of life.
Waste Electrical and Electronic Equipment (WEEE) Directives (2002/96/EC, 2003/108/EC)
Waste Electrical and Electronic Equipment Regulations 2006 (UK)
Etos-Scientific complies with the WEEE directives and regulations. You may contact us for disposal for medical device or equipment that displays the following symbol.
Instructions for Disposal of Waste Equipment by Users in the European Union
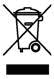 This symbol on the product or its packaging indicates that this product must not be disposed of with other waste. Instead, it is your responsibility to dispose of your waste equipment by handing it over to a designated collection point for the recycling of waste electrical and electronic equipment. The separate collection and recycling of your waste equipment at the time of disposal will help conserve natural resources and ensure that it is recycled in a manner that protects human health and the environment. For more information about where you can drop off your consumer waste equipment for recycling, please contact your local city recycling office or the dealer from whom you originally purchased the product. This symbol on the product or its packaging indicates that this product must not be disposed of with other waste. Instead, it is your responsibility to dispose of your waste equipment by handing it over to a designated collection point for the recycling of waste electrical and electronic equipment. The separate collection and recycling of your waste equipment at the time of disposal will help conserve natural resources and ensure that it is recycled in a manner that protects human health and the environment. For more information about where you can drop off your consumer waste equipment for recycling, please contact your local city recycling office or the dealer from whom you originally purchased the product.
Ethical Dealings
Etos-Scientific expects our employees and suppliers to conduct business in accordance with the highest ethical standards. These include, but are not limited to:
- Compliance with all anti-bribery laws, including the U.S. Foreign Corrupt Practices Act. This includes not offering or providing cash or noncash gifts to any customer to influence them to take or not take a course action or for any other improper purpose
- Not to accept bribes, kickbacks or other similar improper or unlawful payments from anyone, including customers, suppliers and business associates
- Not to use child labor in accordance with local minimum age for employment laws, or under the age for completing compulsory education, whichever is the greatest. We support the use of legitimate workplace apprenticeship programs which comply with all laws and regulations applicable to such apprenticeship programs
- Other prohibited business practices
Health and Safety
Etos-Scientific provides a safe and healthy workplace in compliance with all applicable laws and regulations. We implement the following health and safety management system at our operations:
- A management system has been implemented to identify and control potential hazards and to strive for continuous improvement
- Workplace incidents are reported and investigated and action taken to minimize the potential for future occurrence
- Supplier selection and safety performance are monitored
Privacy
As a global company, Etos-Scientific's business processes go beyond the borders of one country. This globalization requires the availability of communication and information systems across our companies and the worldwide processing and use of information within Etos-Scientific.
Etos-Scientific remains committed to protecting the privacy and confidentiality of personal information about its employees, customers, partners and other identifiable individuals. Uniform practices for collecting, using, disclosing, storing, accessing, transferring or otherwise processing such information assists Etos-Scientific to process personal information fairly and appropriately, disclosing it and/or transferring it only under appropriate circumstances.
Etos-Scientific may create, develop or receive information about patients' health information with our medical devices in a variety of situations, including:
- We provide information or technical support for our products
- We collect information as required by governmental agencies relating to the quality, safety and efficacy of our devices
- We collect, analyze and re-analyze our data in a continuous effort to improve the design, quality and functioning of our devices
- Data may be transferred to an overseas country that ensures an adequate level of protection for the rights and freedoms of data subjects in relation to the processing of personal data. We may also transfer anonymized information in the course of our global operations. All data will be handled in strict accordance with privacy protections safeguards in place.
Etos-Scientific expects our employees and suppliers to respect our customer's explicit and implicit instructions regarding incidental exposure to protected health information, in conjunction of HIPAA privacy regulations in the United States and worldwide with relevant data protection requirements.
Health Insurance Portability and Accountability Act (U.S.)
We understand that most of our U.S. customers are “Covered Entities” under the Health Insurance Portability and Accountability Act (“HIPAA”) privacy regulations to maintain the privacy of all patient information that they create or receive. While Etos-Scientific is not a “Covered Entity”, we recognize the impact that the regulations have on our customers. Etos-Scientific is dedicated to maintaining the privacy of information that we receive, consistent with applicable law and regulations.
EC Directive 95/46/EC (E.U.) and Data Protection Act 1988 (U.K.)
We understand that most of our E.U. and U.K. customers are covered under the EC Directive 95/46/EC and/or Data Protection Act 1998 on the protection of individuals with regard to the processing of personal data and on the free movement. We recognize the impact that these regulations have on our customers. Etos-Scientific is dedicated to maintaining the privacy of information that we receive, consistent with applicable law and regulations.
Quality Assurance
Etos-Scientific management takes the promotion and awareness of regulatory requirements as a management responsibility in the following aspects
- focus on risk management activities and design transfer activities during product development
- specific requirements for documentation and validation of processes for sterile medical devices
- specific requirements for verification of the effectiveness of corrective and preventive actions
We identify key processes using a value chain methodology in order to enable us to define objective measurements, monitor and analyze that our quality objectives are met. Decision about the quality management is made based on recorded data. Our quality management system extends to our suppliers for the publication of specifications that includes documentation of test and control processes.
Our quality management system is used to comply with all local government regulations and certification requirement including ISO 13485:2003 for the implantation of a comprehensive management system in the design and manufacture of medical devices and ISO 9001:2000 for a continuous improvement system, where applicable.
21 CFR 820 Quality System Regulation for Medical Devices (U.S.)
The current Good Manufacturing Practice (GMP) requirements set forth in the Quality System (QS) regulation are promulgated under section 520 of the Food, Drug and Cosmetic (FD&C) Act. They require that domestic or foreign manufacturers have a quality system for the design, manufacture, packaging, labeling, storage, installation, and servicing of finished medical devices intended for commercial distribution in the United States. The regulation requires that various specifications and controls be established for devices; that devices be designed under a quality system to meet these specifications; that devices be manufactured under a quality system; that finished devices meet these specifications; that devices be correctly installed, checked and serviced; that quality data be analyzed to identify and correct quality problems; and that complaints be processed. Thus, the QS regulation helps assure that medical devices are safe and effective for their intended use. The Food and Drug Administration (FDA) monitors device problem data and inspects the operations and records of device developers and manufacturers to determine compliance with the GMP requirements in the QS regulation.
The QS Regulation is contained in Title 21 Part 820 of the Code of Federal Regulations. This regulation covers quality management and organization, device design, buildings, equipment, purchase and handling of components, production and process controls, packaging and labeling control, device evaluation, distribution, installation, complaint handling, servicing, and records.
Medical Devices Directive (93/42/EEC)
Etos-Scientific’s products are generally covered under this directive. We maintain and perform conformity assessment activities against the directive such as:
- Auditing quality system
- Reviewing technical documentation
- Conducting continued surveillance of the quality system
|